1.前言
Drug manufacturers have employed contamination control measures for decades as a core element of good manufacturing practices. Commonly, these are a collection of practices that were developed separately and applied without clear consideration for their interdependence.
药品生产中的污染控制一直以来被作为GMP生产的核心,这些措施通常是一系列单独制定和应用的实践,没有强调他们之间的相互依存关系。
开篇强调了我们需要注意已有的污染控制措施之间的相互关系,且说明污染控制一直是我们GMP生产的核心,那么我们为什么还要考虑CCS策略呢?在我个人以为CCS评估是我们需要注意的重点,都需要说来参与CCS评估看下图。看下文。
CCS策略评估和执行小组(图片来源于网络,仅供学习交流用)
The ongoing evolution of contamination control principles that this document addresses is a shift to a holistic approach, where the practices are designed to work together to achieve proactive contamination control and are evaluated for their collective effectiveness. The holistic approach also demands that contamination control measures be tailored to the specific risks around each individual process。
这一段强调CCS控制策略向整体控制策略演变,旨在实现主动的污染控制并对整体有效性进行评估,同时整体的污染控制策略适应每个单独过程的特定风险。
Many GMP practices are part of a company’s contamination control strategy (CCS) including, for example, how processes and facilities are designed (including cleaning and disinfection), how raw materials and consumables are selected and managed, and how personnel are trained and developed. The CCS is also intended to drive continuous improvement and/or remediation. The success of any one CCS element is intrinsically linked to the others, and the success of the CCS depends upon how well the individual elements work together to reduce the contamination hazards for a specific process (see Figure 3.0-1)。
本段强调了GMP实践中工艺和设施的设计(包括清洁消毒)、原料与耗材的选择及管理、人员培训和提升等均为CCS控制策略的重要组成部分。CCS的目的在于持续改进。我们需要关注各CCS各要素之间的内在联系,CCS的成功在于各个要素相互之间的配合。
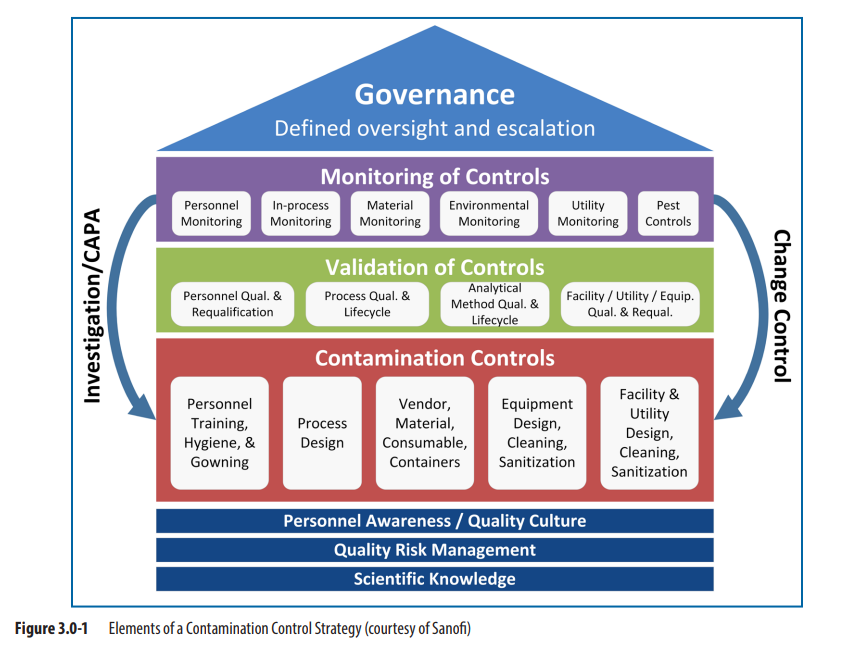
污染控制策略要素图(图片来源于网络,仅供学习交流用)
All drug manufacturers have a CCS that includes a multitude of GMP practices documented across numerous operational procedures and programs. The rationales for those practices are often captured in risk assessments, validations, and technical documents. A CCS record creates an umbrella document that brings the relevant information together so it can be understood and evaluated holistically. All CCS documents should summarize the contamination control practices, along with the underlying rationales, and reference the supportive procedures and reports. This technical report outlines how to implement an effective CCS using a holistic approach, highlights a selection of best practices, and identifies outdated practices and mindsets. The document opens with a conceptual discussion of the various CCS elements (Section 3.0) with the understanding that all drug manufacturing firms will have these elements in various forms and levels of maturity. The many existing guidances and standards for specific CCS elements, for example, critical utility control, gowning practices, and process hold times, are referenced in Section 14.0. This technical report does not replace or reiterate those de tailed documents; rather it acts as a roadmap. Appendices are also included that provide valuable case studies and practical examples as well as a template for creating a CCS document (see Section 18.0).
这一段强调了CCS文件是一个综括性的文件,将我们日常的污染控制措施及信息进行汇总,便于整体理解和评估。其支持性材料仍为我们日常的风险评估、验证和相关技术文件(如众多的操作规程和操作程序)等。
然后强调了三章:3.0是对CCS控制策略概念性的讨论;14.0提到了CCS控制的指南和标准;18.0提供CCS研究实例和CCS文件模板。
2.目的
PDA Technical Report No. 90: Contamination Control Strategy Development in Pharmaceutical Manufacturing Technical Report provides guidance on how to establish an effective CCS for either a new or existing drug manufacturing facility or process.
目的说到对新的或现有的药品生产设施和工艺建立有效的CCS提供指导,对于我们已有的污染控制措施的公司来说需要建立该文件进行整体评价与持续改善,我们需要将CCS控制策略提高到与工艺控制策略对等的高度。对于工艺控制策略的研究可以指导我们罗氏CCS控制策略,我们并不是从零开始。

工艺控制策略与污染控制策略(图片仅供学习交流用)
而对于新公司来说应更具质量源于设计(QbD)的原则,基于经验和工艺进行设计。
3.范围
This document focuses on contamination control practices against microbial and other adventitious agents, pyrogens such as endotoxin, and foreign particulate matter foreign particulate matter in the manufacture of sterile drugs, low bioburden drug substances, and nonsterile drugs that are vulnerable to contamination. Yet, the principles presented in this technical report can be applied more widely to any drug manufacturing or compounding process.
Secondary and tertiary packaging considerations, chemical contaminants including product cross contamination, and inherent particulate matter are out of the scope of this technical report.
范围强调了无菌药品、低生物负载物质和易受污染的非无菌药品,个人理解这三类会被重点关照,其他的药品生产和配制过程也要落实。
范围明确了研究的控制意图为:
1)无菌保证
2)生物负载控制/低生物负载
3)内毒素/热原控制
4)外来异物控制
其中化学污染(包括交叉污染)、二级和三级包装的考量及固有微粒物质并不在研究范围。
4.关于术语和缩略语
1)行动限
对于行动限需要进行调查、产品影响评估并基于调查结果采取纠正措施。其中对产品的影响评估个人以为后续我们需要注意对产品的影响评估。
Action Limit:An established value that, when exceeded, indicates a process is outside of its normal operating conditions. A response to such an excursion requires a documented investigation, product impact assessment, and corrective actions based on the results of that investigation.
2)警戒限
关于警戒限很多人只关注了其预警的作用,往往忽略仍需适当的进行文件调查和可能需要制定措施这一点。
Alert Level:An established value that, when exceeded, pro vides an early warning of a potential drift from the normal operating conditions and validated state. This type of warning does require an appropriate documented investigation (e.g., trend analysis) and may require corrective actions de pending on the results of the investigation.
3)消除污染
这个概念对于出生于成熟企业的我来说有一点模糊,特别是消除污染方法的筛选这一点,很多时候我们都是成熟的方法,到了新地方多数照搬以前的策略,缺少因地制宜的思考。
Decontamination: The overall process of removal or reduction of any contaminants (chemical residue or micro organisms) from an area, processing equipment, or person. The method of decontamination used (e.g., cleaning, disinfection, sterilization) should be chosen and validated to achieve a level of cleanliness appropriate to the intended use of the item decontaminated。
4)A级送风
这对我来说也是一个新概念,以前总是强调各种洁净级别,但是从未考虑A级送风,例如整衣层流我们日常使用控制级别可以时C级,但是其本身需要具备A级送风能力。基于这个概念,个人以为有些所谓的A级区域我们可以进行松绑。
Grade A Air Supply:Air which is passed through a filter qualified as capable of producing grade A total particle quality air, but where there is no requirement to perform continuous total particle monitoring or meet grade A viable monitoring limits.
5)RABS
RABS内表面需要使用杀孢子剂进行消毒和杀菌处理和几乎不要打开RABS的门这两点是我关注的重点。
Restricted Access Barrier System (RABS):System that provides an enclosed, but not fully sealed, environment meeting defined air quality conditions (for aseptic processing grade A), and using a rigid-wall enclosure and integrated loves to separate its interior from the surrounding cleanroom environment. The inner surfaces of the RABS are disinfected and decontaminated with a sporicidal agent. Operators use gloves, half suits, RTPs and other integrated transfer ports to perform manipulations or convey materials to the interior of the RABS. Depending on the design, doors are rarely opened, and only
under strictly pre-defined conditions.
6)缩略语
API Active pharmaceutical ingredient 药物活性成分
CCS Contamination control strategy 污染控制策略
FMEA Failure Modes and Effects Analysis 失败模式和影响分析
HACCP Hazard Analysis and Critical Control Points 危害分析与关键点控制
HVAC Heating, ventilation, and air conditioning 供暖、通风和空调
RABS Restricted Access Barrier System 限制进入屏障系统
QRM Quality risk management 质量风险管理
5.小结
学习完前言基于以往经验我以为我们先建立CCS管理程序,然后执行污染控制评估并指导污染控制改进,最终编制CCS,过程中做好人员的培训,实现CCS监控与持续改进,最终实现我们质量文化的提升。
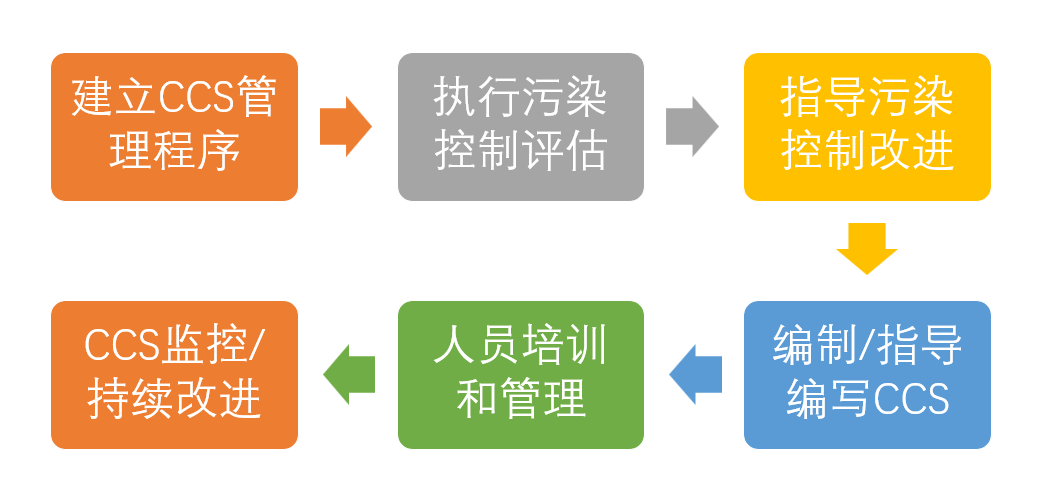
以上是个人学习心得,受限于个人认知,难免有错漏之处,欢迎各位老师交流指正。

