写在前面的话:
该部分首先讲了温度、pH及盐浓度等因素对LER现象的影响,并确定了他们与LER现象的关系。该部分除指导我们需要控制温度外,该部分的研究内容还可以作为我们分析LER研究数据时作为参考。
最后文中关于LPS存在的三个阶段值得借鉴。其他关于涡旋对LER的影响等细节详细内容见下文。
8.4Case Study 4: Factors Affecting Low Endotoxin Recovery实例研究4:影响低内毒素回收率的因素
Reduction of reference standard endotoxin activity was kinetically analyzed under LER conditions containing a chelating agent and a detergent and was considered an apparent first-order reaction. Temperature, pH, and salt concentrations affected the reduction rates of reference standard endotoxin activity; temperature appeared to be the most important factor affecting LER. Components of LER matrices, such as citrate and polysorbate 20, showed similar LER effect at the concentrations commonly used. Phosphate showed negative correlation against the half-life of reference standard endotoxin in LER solutions. Lower temperature, lower pH, and a higher salt concentration are preferable to avoid LER in a hold-time study. Effects of dilution methods were also evaluated and the dilution method with 2 mM magnesium was considered to be suitable for the detection of reference standard endotoxin activity in LER solutions.
在含有螯合剂和清洁剂的LER条件下对内毒素参考品(RSE)活性降低进行了动力学研究分析,研究认为这是明显的一步反应。温度、pH值和盐浓度影响内毒素参考品(RSE)活性的降低速率,其中温度似乎是影响LER的最重要因素。LER基质组分(如柠檬酸盐和聚山梨酯20)在常用浓度下显示相似的LER效应。在LER溶液中磷酸( 磷酸)盐与内毒素参比标准品的半衰期呈负相关。在保持时间研究中较低的温度、较低的pH值和较高的盐浓度更有利于避免LER。同时对稀释方法的影响进行了评价,在LER溶液中使用2 mM镁进行稀释有利于内毒素参考品活性的检测。
磷酸)盐与内毒素参比标准品的半衰期呈负相关。在保持时间研究中较低的温度、较低的pH值和较高的盐浓度更有利于避免LER。同时对稀释方法的影响进行了评价,在LER溶液中使用2 mM镁进行稀释有利于内毒素参考品活性的检测。
注释1:化学动力学(chemical kinetics),也称反应动力学、化学反应动力学,是研究化学过程进行的速率和反应机理的物理化学分支学科。它的研究对象是性质随时间而变化的非平衡的动态体系。化学动力学往往是化工生产过程中的决定性因素。通过化学动力学的研究,可以知道如何控制反应条件,提高主反应的速率,增加产品产量,抑制副反应的速率,减少原料消耗,减少副产物,提高纯度,提高产品质量。
8.4.1Introduction引言
LER-type phenomena have been observed before. For example, reference standard endotoxin (RSE) or control standard endotoxin (CSE) activity was decreased by trace amounts of certain metal cations, such as aluminum (1), iron (1,2), and gallium (1,3), and some antibiotics (3). Tsuchiya developed a modified spiking method to observe the effect of a sample on endotoxin detection (1), and Fujita, et al., applied the method to their products (3). They demonstrated that the addition of endotoxin, after mixing the sample, and Limulus amebocyte lysate (LAL) avoided the effects of those substances on spiked endotoxin. The modification of spiking methods suggested that those substances altered the potency of spiked endotoxin, and that there was no inhibition of the substances to the LAL activation.
以前曾观察到类似于LER现象,如微量的铝(1)、铁(1,2)和镓(1,3)等金属阳离子以及一些抗生素(3)会降低内毒素参考品(RSE)或内毒素工作标准品(CSE)的活性。Tsuchiya开发了改良的加标方法用于观察样品对内毒素检测的影响[1],Fujita等人将该方法应用于其产品[3]。他们的研究证实将待测样品与鲎试剂混合后加入内毒素可避免这些物质(前面的金属离子和抗生素)对加标内毒素的影响。经改良的加标方法表明这些物质改变了加标内毒素的效价,但这些物质对鲎试剂的活性无影响。
In LER cases, the activity of the spiked lipopolysaccharide (LPS) to undiluted products was not recovered by a common treatment, such as simple dilution with water, even though the diluted sample did not show inhibition to the bacterial endotoxins test (BET) (4). This suggests that LER is a change in endotoxin activity caused by the product and is not inhibition of LAL activation by the product. Typical LER is caused by a matrix containing a chelating agent and a detergent (4,5); several mechanisms have been proposed (6,7). Some factors affecting LER were reported (7,8), but no study has described a kinetic analysis on LER under different conditions.
在LER研究案例中,即使稀释样品对细菌内毒素试验(BET)未表现出抑制作用,未经稀释样品中的加标脂多糖(LPS)并不能通过普通处理((如用水进行稀释)提高其回收率(4)。这表明LER是产品引起的内毒素活性变化而不是产品抑制LAL活性。典型的LER是由含有螯合剂和洗涤剂的基质引起的(4,5);人们已经提出了几种可能的机制(6,7)。一些影响LER因素可见于报告(7,8),但在不同条件下LER的动力学分析还未被深入研究。
Platco et al., reported that a non-interfering concentration of cation buffer unmasked CSE/LPS in their monoclonal antibody products containing citrate and polysorbate (9). They considered that the dilution of their products, with cation buffer containing magnesium, resurrected the CSE activity. The results of this study confirmed the effectiveness of the magnesium dilution in the LER study and found that spiked RSE activity in LER solutions was decreased during the dilution with water, even though its activity was maintained in the original LER solutions for more than two weeks at 2 °C-5 °C (10). This finding provided a new aspect of the mechanism of LER.
Platco et al.报告,不干扰浓度的阳离子缓冲液可消除含柠檬酸盐和聚山梨酯的单克隆抗体( 抗体)产品中的CSE/LPS受到的屏蔽(9)。他们认为用含镁的阳离子缓冲液稀释其产物可使CSE恢复活性。本研究结果证实了LER研究中镁稀释液的有效性,并发现在用水稀释期间,LER溶液中的加标RSE活性降低,即使其活性在2 ℃-5 ℃下在原始LER溶液中保持2周以上(10)。这一发现为LER的机制提供了一个新的方向。
抗体)产品中的CSE/LPS受到的屏蔽(9)。他们认为用含镁的阳离子缓冲液稀释其产物可使CSE恢复活性。本研究结果证实了LER研究中镁稀释液的有效性,并发现在用水稀释期间,LER溶液中的加标RSE活性降低,即使其活性在2 ℃-5 ℃下在原始LER溶液中保持2周以上(10)。这一发现为LER的机制提供了一个新的方向。
In this study, real-time activity changes of RSE were measured under LER conditions. Even though the LER mechanism is considered to involve at least two steps, chelating reaction and detergent effect (6,7), the kinetics of RSE activity change can be analyzed by an apparent first-order reaction, and the half-life of RSE was calculated from the reaction rate constant k for each LER condition (11). The information on the half-life of RSE provides the outcome of factors affecting LER, which is useful to design the hold-time study conditions. Dilution methods were also evaluated with LER solutions. The direct assay method and the magnesium dilution method were developed to measure the activity in original LER solutions, and the magnesium dilution method was considered a suitable dilution method for LER solutions (10).
本研究在LER条件下测定RSE的实时活性变化。尽管LER机制被认为至少涉及螯合反应和去污剂效应两个步骤(6,7),但RSE活性变化的动力学可以通过表观一级反应进行分析,并根据每个LER条件下的反应速率常数k计算RSE的半衰期(11)。RSE半衰期的信息可反映出影响LER各因素对LER现象的影响结果,这有助于设计保持时间的研究条件。还使用LER溶液评价稀释方法。建立直接测定法和镁稀释法来测定原始LER溶液中的活性,镁稀释法被认为是LER溶液的合适稀释方法[10]。
注释2:
表观一级反应:由传质控制和界面化学控制的、速率经验方程与浓度的一次方成正比的一类反应。
8.4.2Materials and Methods材料和方法
8.4.2.1Sample Preparation样品制备
Stock solutions were prepared from reagent-grade chemicals and were then filtered with positively charged 0.2 mm filters (Acrodisc® Syringe Filter 0.2 mm Posidyne® Membrane, Pall Life Science, Ann Arbor, MI). Endotoxin was not detected in the stock solutions by the LAL test. LAL Reagent Water (LRW, Charles River, Charleston, SC) was used, and 5% sodium chloride was purchased from B/Braun (5% Sodium Chloride Injection USP, Irvine, CA). A solution containing 111% of target concentrations of the components were prepared from stock solutions and used as a base LER solution. The concentrations of the components were adjusted at 100% by addition of one-tenth volume of an RSE solution for the initiation of LER reaction. The USP reference standard endotoxin (RSE, 10,000 USP endotoxin units (EU) per vial) was reconstituted with 5 ml LRW and diluted with LRW to preferable concentrations.
使用试剂级化学品制备储备液,然后用带正电荷的0.2 mm过滤器过滤(Acrodisc®针头式过滤器0.2 mm Posidyne®膜,Pall Life Science,Ann Arbor,MI)。通过LAL试验在贮备液中未检出内毒素。使用LAL试剂水(LRW,Charles River,Charleston,SC),5%氯化钠( 氯化钠)购自B/Braun(5%氯化钠注射液(
氯化钠)购自B/Braun(5%氯化钠注射液( 氯化钠注射液)USP,Irvine,CA)。使用储备液制备含111%目标浓度组分的溶液,并用作基础LER溶液。通过在LER基础溶液与RSE溶液按9:1(体积比)混匀,将目标组分浓度调节至100%,以启动LER反应。采用5 mL LRW复溶美国药典对照品内毒素(RSE,10,000 USP内毒素单位(EU)/瓶),并用LRW稀释至最佳浓度。
氯化钠注射液)USP,Irvine,CA)。使用储备液制备含111%目标浓度组分的溶液,并用作基础LER溶液。通过在LER基础溶液与RSE溶液按9:1(体积比)混匀,将目标组分浓度调节至100%,以启动LER反应。采用5 mL LRW复溶美国药典对照品内毒素(RSE,10,000 USP内毒素单位(EU)/瓶),并用LRW稀释至最佳浓度。
注释3:
1.Reagent:试剂级,与CP级相近。化学纯(CP,蓝标签)(三级品)主成分含量高、纯度较高,存在干扰杂质,适用于化学实验和合成制备。
2.内毒素带负电,使用带阴离子吸附柱可以过滤可以除内毒素(这个是参考离子交换色谱蛋白纯化技术)。
3.离子交换色谱是蛋白纯化技术中常用的一种纯化方法,其原理是指被分离物质所带的电荷可与离子交换剂所带的相反电荷结合,这种带电分子与固定相之间的结合作用是可逆的,在改变pH或者用逐渐增加离子强度的缓冲液洗脱时,离子交换剂上结合的物质可与洗脱液中的离子发生交换而被洗脱到溶液中。由于不同物质的电荷不同,其与离子交换剂的结合能力也不同,所以被洗脱到溶液中的顺序也不同,从而被分离出来。
4.通过111%的浓度的目标溶液按9:1的比例得到近似100%的目标溶液,这种稀释方法值得借鉴。
8.4.2.2 Sample Dilution Methods and Measurement of Endotoxin样品稀释方法和内毒素的测定
Endotoxin activity was measured by the kinetic chromogenic (KCA) or the kinetic turbidimetric (KTA) LAL method. LAL reagents used were Endochrome-KTM (Charles River) for KCA and KTA2TM (Charles River) for KTA. The LAL reagents were reconstituted with the endotoxin-specific buffer (BG120, Charles River) to eliminate possible glucan interference. Endotoxin standard curves were established by logarithmic plotting of endotoxin concentrations versus onset times (reaction time to obtain a 5% decrease of transmittance of a reaction mixture). Quadratic regression was used to calculate endotoxin concentrations in samples. The dilution methods are as follows:
通过动态显色(KCA)或动态浊度法(KTA)LAL测定内毒素活性。使用的LAL试剂为Endochrome-KTM(Charles River)(用于KCA)和KTA2TM(Charles River)(用于KTA)。用内毒素特异性缓冲液(BG120,Charles River)复溶LAL试剂,以消除可能的葡聚糖干扰。以内毒素浓度对起始时间(使反应混合物的透光度下降5%所需的反应时间)作图,建立内毒素标准曲线。使用二次回归计算样品中的内毒素浓度。稀释方法如下:
Direct Assay Method: A 0.01 mL of sample was added to a reaction tube containing 0.1 mL LAL and 0.1 mL LRW, and the reaction mixture was set on a tube reader (ToxinometerTM, Wako Pure Chemical Industries, Ltd., Osaka, Japan) to perform the LAL test.
直接试验方法:将0.01 mL样品加入含0.1 mL LAL和0.1 mL LRW的反应管中,并将反应混合物置于酶标仪(ToxinometerTM,Wako Pure Chemical Industries,Ltd.,Osaka,Japan)上进行LAL试验。
•Water Dilution Method: Samples were diluted with LAL Reagent Water (Charles River) in polypropylene test tubes (Falcon 352054, BD Biosciences, Bedford, MA). Endotoxin activity in the diluted samples were measured by the LAL test.
水稀释法:样品在聚丙烯试管(Falcon 352054,BD Biosciences,Bedford,MA)中用LAL试剂水(Charles River)稀释。通过LAL试验测定稀释样品中的内毒素活性。
•Magnesium Dilution Method: Samples were diluted with 2 mM magnesium sulfate solution in polypropylene test tubes. Endotoxin activity in the diluted samples was measured by the LAL test.
镁稀释法:样品在聚丙烯试管中用2 mM硫酸镁( 硫酸镁)溶液稀释。通过LAL试验测定稀释样品中的内毒素活性。
硫酸镁)溶液稀释。通过LAL试验测定稀释样品中的内毒素活性。
8.4.2.3Measurement of Reduction Rates of RSE Activity in LER Solutions LER溶液中RSE活性降低率的测量
A base LER solution in a polystyrene test tube was put on an aluminum block heater set at a target temperature and kept for 15 min to 1 h before starting the reaction. One-tenth of an RSE dilution was added to the LER solution to start the reaction. The LER solution was vortexed for approximately 15s, and a sample at T0 was taken for the initial assay. Endotoxin activity was measured by the direct assay method with preferable timing. The direct assay method could technically be performed as frequently as once per minute, but the timing was adjusted to the RSE activity reduction rate. In most of the cases, assays were performed every 10 min. Reduction rates of RSE activity were obtained from slopes derived by plotting incubation time versus the natural logarithm of reciprocals of endotoxin recovery.
将聚苯乙烯试管中的基础LER溶液置于设定为目标温度的铝块加热器上,并在开始反应前保持15 min至1 h。向LER溶液中加入十分之一的RSE稀释液,开始反应。涡旋LER溶液约15 s,并在T0时采集样本用于初始分析。在最佳时间点使用直接测定法测定内毒素活性。直接测定法在技术上可以每分钟进行一次测定,但按RSE活性降低率来调整检测时间。在大多数情况下,每10 min进行一次测定。通过绘制孵育时间与内毒素回收率倒数自然对数的斜率,获得RSE活性的降低率。
注释4:基于检测对象进行研究。
8.4.2.4Analytical Theory and Assumption分析理论和假设
Though LER phenomenon is supposed to be two-step reaction (7), the RSE activity change was simply assumed to be a first-order reaction from active state LPS (LPSa) to inactive state LPS (LPSi).
虽然LER现象被认为是两步反应(7),但RSE活性变化被简单地假定为从活化态LPS(LPSa)到非非活化态态LPS(LPSi)的一级反应。
LPSa→ LPSi
The rate of reaction is expressed as:反应速率表示为
v = -d [LPSa]/dt = k[LPSa]
where v is reaction velocity and k is reaction rate constant.
其中v是反应速度,k是反应速率常数。
The above equation can be written equivalently as:上述公式可等同于:
-d (c-x)/dt = dx/dt = k(c-x)
where c is the initial concentration of LPSa and x is a concentration of reacted LPSa at time t.
When t = 0 and x = 0, the integrated equation is:
其中c是LPSa的初始浓度,x是时间t时反应LPSa的浓度。当t = 0和x = 0时,积分方程为:
k = (1/t )* ln(c/(c-x))
modify this equation:修改此公式
ln (c/(c-x)) = kt (Eq. 1)
Where LPS activity is expressed as recovery ratio, c = 1 and c-x = endotoxin recovery at time t. therefore, k can be obtained as a slope of plotting t versus the natural logarithm of 1/endotoxin recovery. If this assumption is not correct, this plotting should not be linear. The reaction rate constant k was used as the reduction rate of RSE activity under the analyzed LER condition.
其中LPS活性表示为回收率,c = 1和c-x = t时的内毒素回收率。因此,k可表示为绘制t与1/内毒素回收率自然对数的斜率。如果该假设不正确,则该图不应呈线性。以反应速率常数k作为分析LER条件下RSE活性的降低速率。
8.4.2.5Calculation of Half-Life of RSE Activity in LER Solutions LER溶液中RSE活性半衰期的计算
The half-life (HL) of spiked endotoxin in an LER solution was defined as the reduction time required at a given condition to reduce by 50% the spiked endotoxin activity. Time required to obtain 50% activity was calculated from a reaction rate constant k and used as an HL value.
LER溶液中加标内毒素的半衰期(HL)定义为在给定条件下使加标内毒素活性降低50%所需的降低时间。根据反应速率常数k计算获得50%活性所需的时间,并用作HL值。
Modify Eq. 1:修改方程
t = (1/k) ln(c/(c-x))
HL is a time when c = 1 and x = 0.5:
HL是c = 1和x = 0.5时的时间:
HL = (1/k) ln(1/0.5)= (1/k) ln 2 = 0.693/k
8.4.3Results and Discussion结果和讨论
8.4.3.1Suitability of Analysis as First-Order Reaction作为一级反应的分析方法适用性
Table 8.4.3.1-1 and Table 8.4.3.1-2 show the results of calculation of the reduction rate k. Correlation coefficients r were higher than 0.9 instead of three cases that showed less than 0.1 for k. Because most of the correlation coefficients were higher than 0.9, good linearity was indicated and, therefore, the RSE reduction was considered an apparent first-order reaction. Thethree cases that showed low correlation coefficients had no impact on the conclusion, because they were considered to be caused by gradual slopes of the regression curves. Suitability of the assumption allowed calculation of the reaction rate constants k and the half-life of RSE activity. The magnitude of effects on the half-life of RSE activity in LER solutions was compared to the factors affecting LER. Temperature, pH, salt concentrations, and the components in LER matrices, i.e., citrate, phosphate, and polysorbate 20, were selected to evaluate their effects on LER.
在表8.4.3.1-1和表8.4.3.1-2中给出了下降率k的计算结果。相关系数r大于0.9,而不是k小于0.1的三种情况。因为大多数相关系数大于0.9,表明具有良好的线性,因此认为RSE下降是明显的一级反应。低相关系数的3例对结论无影响,因为认为其是由回归曲线的渐变斜率引起的。假设的适用性允许计算反应速率常数k和RSE活性的半衰期。将对LER溶液中RSE活性半衰期的影响幅度与影响LER的因素进行比较。选择温度、pH值、盐浓度和LER基质中的组分(即柠檬酸盐、磷酸盐和聚山梨酯20)评价其对LER的影响。
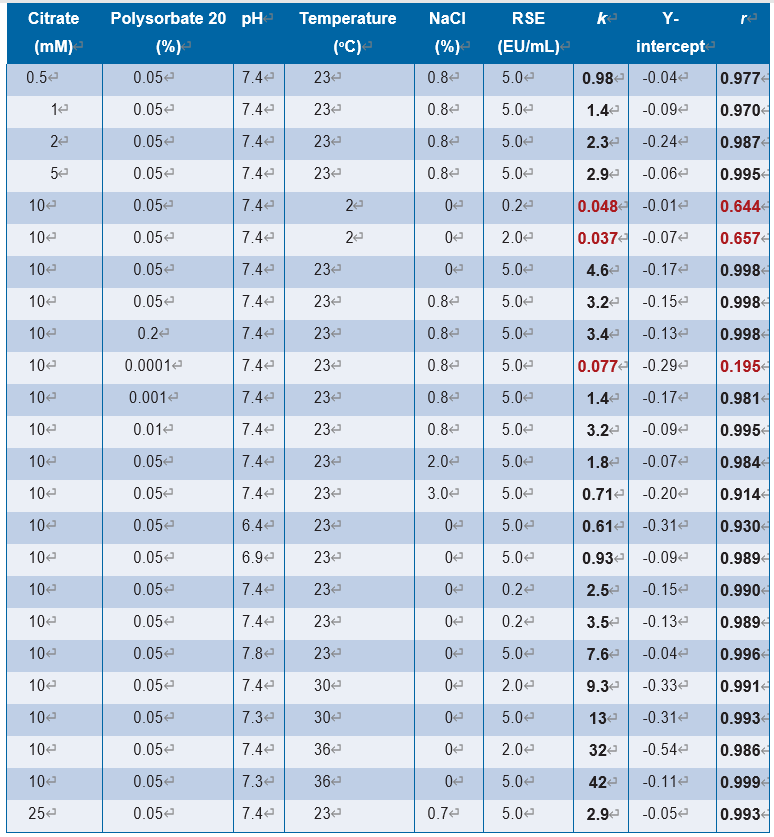
Table 8.4.3.1-2 Kinetic parameters for RSE activity change in LER solutions containing phosphate表8.4.3.1-2含磷酸盐的LER溶液中RSE活性变化的动力学参数(图片来源于网络,仅供学习交流用)
Plotting incubation time versus the natural logarithm of reciprocals of endotoxin recovery provided the kinetic parameters, reaction rate constant k, y-intercept, and correlation coefficients r.
绘制孵育时间与内毒素回收率倒数的自然对数图,提供动力学参数、反应速率常数k、y轴截距和相关系数r。
8.4.4Effects of Temperature, pH, and Salt Concentration on LER 温度、pH值和盐浓度对LER的影响
Figure 8.4.4-1 shows the effect of temperature on the half-life of RSE activity under an LER condition. Higher temperature produced a shorter half-life of RSE activity. A negative linear correlation was observed between temperature and the logarithm of half-life of RSE activity. Reich, et al., pointed out that the LER reaction was temperature-dependent (7). In this study, the temperature effect was kinetically analyzed. Temperature showed a strong effect on the half-life of RSE activity in LER solutions. The results agreed with the observation by Reich, et al., and provided an estimation of the difference of the half-life of RSE activity caused by different storage temperatures. For example, the half-life of RSE activity at 2 oC was about 100 times longer than that at 25 oC. This indicates that temperature should be strictly controlled for the hold-time studies. Bolden, et al., reported that mixing time of LER solutions affected endotoxin recovery, and a shorter mixing time (1 min) showed better endotoxin recovery than a longer mixing time (15 min) (8). Their samples were stored at 2 oC-8 oC, but the temperature was elevated during the mixing, which could explain lower endotoxin recovery in the experiments (9). Because the temperature of LER solutions cannot be ignored in hold-time studies, previous data should be reanalyzed considering the operating temperature. The conditions of LER studies should be designed carefully, especially on the temperature control, because temperature strongly affects the half-life of RSE activity under LER conditions.
如图8.4.4-1所示,在LER条件下温度对RSE活性半衰期的影响。温度越高,RSE活性的半衰期越短。温度与RSE活性半衰期的对数呈线性负相关。Reich等指出LER反应具有温度依赖性[7]。在本研究中,对温度效应进行了动力学分析。温度对LER溶液中RSE活性的半衰期有较强的影响。结果与Reich等人的观察结果一致,并提供了不同储存温度引起的RSE活性半衰期差异的估计值。例如,2℃时RSE活性的半衰期约是25℃时的约100倍。这表明在放置时间研究中应严格控制温度。Bolden等报道LER溶液的混合时间影响内毒素回收率,较短的混合时间(1 min)比较长的混合时间(15 min)表现出更好的内毒素回收率[8]。将样品储存在2℃-8℃,但混合过程中温度升高,这可以解释实验中内毒素回收率较低的原因[9]。由于在放置时间研究中不能忽略LER溶液的温度,因此对先前数据进行分析前应考虑操作温度。LER研究条件应进行精心设计,特别是在温度控制上,因为温度强烈影响LER条件下RSE活性的半衰期。
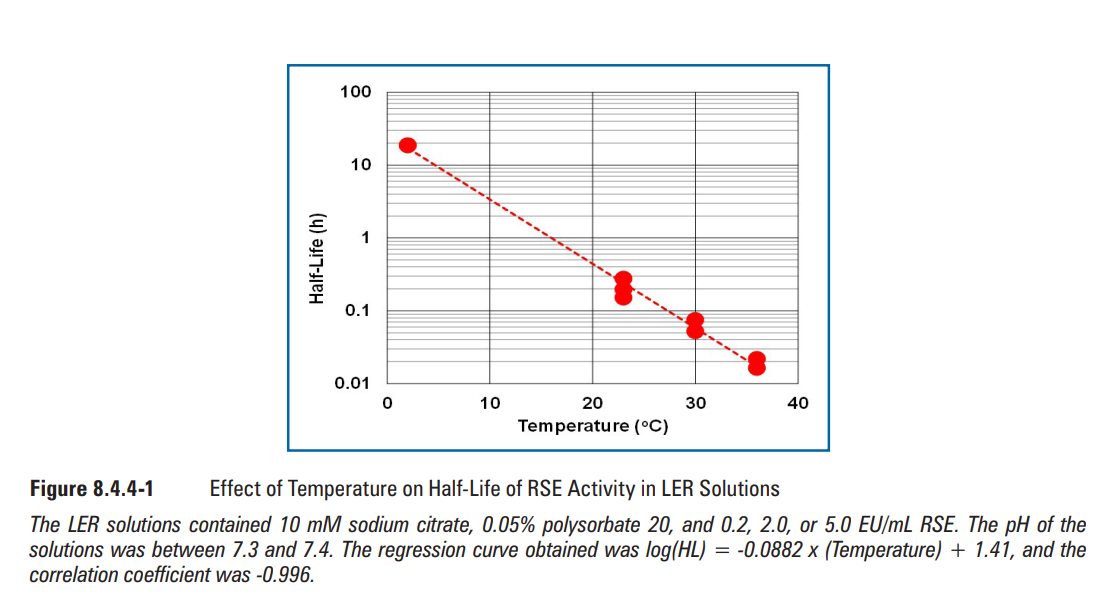
图8.4.4-1温度对LER溶液中RSE活性半衰期的影响(图片来源于网络,仅供学习交流用)
LER溶液含有10 mM柠檬酸钠、0.05%聚山梨酯20以及0.2、2.0或5.0 EU/mL RSE。溶液pH值在7.3 ~ 7.4之间,回归曲线为log(HL)=-0.0882x(温度)+ 1.41,相关系数为-0.996。
As shown in Figure 8.4.4-2, higher pH produced a shorter half-life of RSE activity. The slope with citrate buffer was slightly steeper than that with phosphate buffer, and both buffer components showed a negative linear correlation between pH and the logarithm of half-life of RSE activity. It was reported that the pH of the sample affected LER (7). The pH effect obtained in this study confirmed that higher pH provided a faster decrease of RSE activity. This suggested that the chelating reaction is very important for the LER process because pH affects the chelating reaction.
如图8.4.4-2所示,pH越高,RSE活性的半衰期越短。柠檬酸盐缓冲液的斜率比磷酸盐缓冲液略陡峭,两种缓冲液组分均显示pH值与RSE活性半衰期对数呈线性负相关。据报告,样本的pH值影响LER(7)。本研究中获得的pH值效应证实,较高的pH值可更快地降低RSE活性。这表明螯合反应对LER过程非常重要,因为pH影响螯合反应。
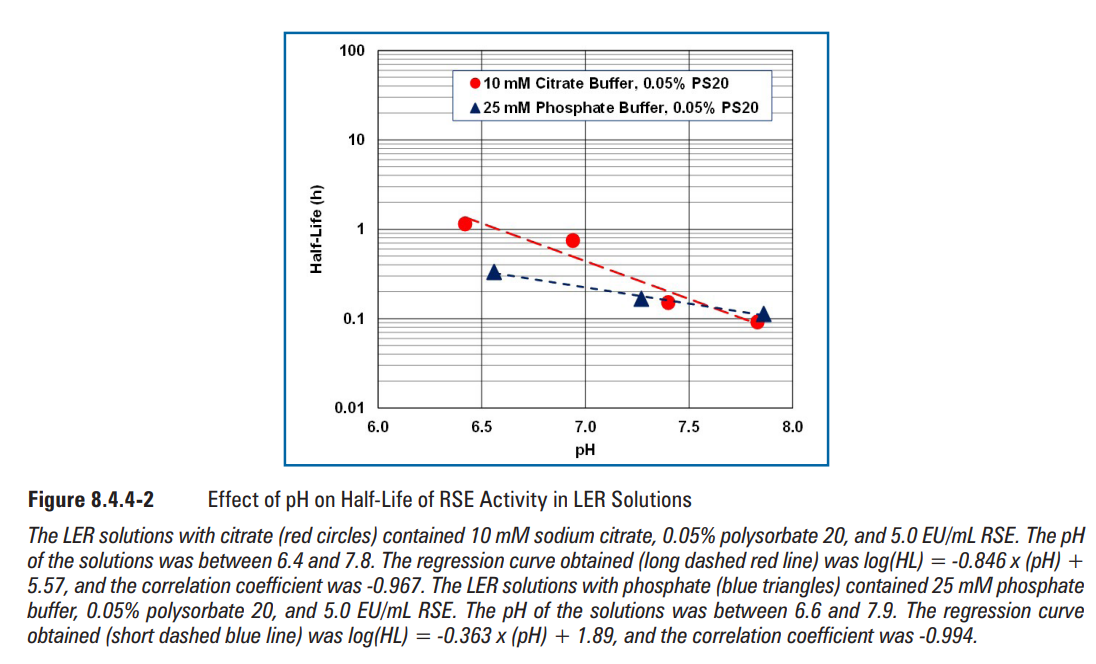
图8.4.4-2pH值对LER溶液中RSE活性半衰期的影响(图片来源于网络仅供学习交流用)
含柠檬酸盐的LER溶液(红色圆圈)含10 mM柠檬酸钠、0.05%聚山梨酯20和5.0 EU/mL RSE。溶液pH值在6.4~7.8之间,所得回归曲线(红色长虚线)为log(HL)=-0.846x(pH)+ 5.57,相关系数为-0.967。含磷酸盐的LER溶液(蓝色三角形)含25 mM磷酸盐缓冲液、0.05%聚山梨酯20和5.0 EU/mL RSE。溶液的pH值在6.6~7.9之间,所得回归曲线(蓝色短虚线)为log(HL)=-0.363x(pH)+ 1.89,相关系数为-0.994。
Lower sodium chloride concentrations produced a shorter half-life of RSE activity (Figure 8.4.4-3). A positive linear correlation was observed between salt concentration and the logarithm of half-life RSE activity. Higher salt concentrations increased the half-life of RSE activity. Biopharmaceutical products usually contain saline, which can provide them less LER effects than water-based products.
氯化钠浓度越低,RSE活性的半衰期越短(图8.4.4-3)。盐浓度与半衰期RSE活性的对数呈正线性相关。较高的盐浓度增加了RSE活性的半衰期。生物制药产品通常含有盐溶液,与水基产品相比,盐溶液可以提供更少的LER效应。
图8.4.4-3盐浓度对LER溶液中RSE活性半衰期的影响(图片来源于网络仅供学习交流用)
LER溶液含10 mM柠檬酸钠、0.05%聚山梨酯20、5.0 EU/mL RSE和0-3%氯化钠。溶液的pH值为7.4.得到的回归曲线为log(HL)= 0.268x(NaCl)-0.873,相关系数为0.986。
8.4.5Effects of Matrix Components on LER at Relevant Concentrations 相关浓度下基质组分对LER的影响
Effects of matrix components on RSE activity in LER solutions were observed at concentrations commonly used in biopharmaceutical products. Sodium citrate (Figure 8.4.5-1), sodium phosphate (Figure 8.4.5-2), and polysorbate 20 (Figure 8.4.5-3) were chosen as the matrix components. The concentrations commonly used for sodium citrate, phosphate buffer, and polysorbate 20 were 5-25 mM, 10-25 mM, and 0.01%-0.2%, respectively. Sodium citrate and polysorbate 20 showed a similar half-life of RSE at the concentrations commonly used. The half-life of RSE activity was prolonged by further reduction of the concentrations of these components. Even though longer half-life was observed at the concentrations lower than that commonly used, these concentrations may not be relevant for biopharmaceutical formulations. A linear correlation was observed in the logarithmic plotting of phosphate buffer concentration versus half-life of RSE activity. Citrate produces a chelate effect and removes divalent cations from LPS. Phosphate is not a chelating agent, but competitively removes divalent cations from LPS. Therefore, citrate is more effective in removing divalent cations from LPS. The difference between the slopes may be caused by the difference in the strength of the agents in divalent-cation removal from LPS.
在生物制药产品中常用的浓度下,观察基质组分对LER溶液中RSE活性的影响。选择枸橼酸( 枸橼酸)钠(图8.4.5-1)、磷酸钠(图8.4.5-2)和聚山梨酯20(图8.4.5-3)作为基质组分。柠檬酸钠、磷酸盐缓冲液和聚山梨酯20的常用浓度分别为5-25 mM、10-25 mM和0.01%-0.2%。柠檬酸钠和聚山梨酯20在通常浓度下的RSE的半衰期相似。通过进一步降低这些组分的浓度可延长RSE活性的半衰期。尽管在低于常用浓度下观察到半衰期更长,但这些浓度可能与生物制剂无关。在磷酸盐缓冲液浓度与RSE活性半衰期的对数图中观察到线性相关。柠檬酸盐可产生螯合作用,并去除LPS中的二价阳离子。磷酸盐不是螯合剂,但也可竞争性去除LPS中的二价阳离子。因此,枸橼酸能更有效地去除LPS中的二价阳离子。斜率之间的差异可能是由于从LPS中去除二价阳离子的试剂强度差异所致。
枸橼酸)钠(图8.4.5-1)、磷酸钠(图8.4.5-2)和聚山梨酯20(图8.4.5-3)作为基质组分。柠檬酸钠、磷酸盐缓冲液和聚山梨酯20的常用浓度分别为5-25 mM、10-25 mM和0.01%-0.2%。柠檬酸钠和聚山梨酯20在通常浓度下的RSE的半衰期相似。通过进一步降低这些组分的浓度可延长RSE活性的半衰期。尽管在低于常用浓度下观察到半衰期更长,但这些浓度可能与生物制剂无关。在磷酸盐缓冲液浓度与RSE活性半衰期的对数图中观察到线性相关。柠檬酸盐可产生螯合作用,并去除LPS中的二价阳离子。磷酸盐不是螯合剂,但也可竞争性去除LPS中的二价阳离子。因此,枸橼酸能更有效地去除LPS中的二价阳离子。斜率之间的差异可能是由于从LPS中去除二价阳离子的试剂强度差异所致。
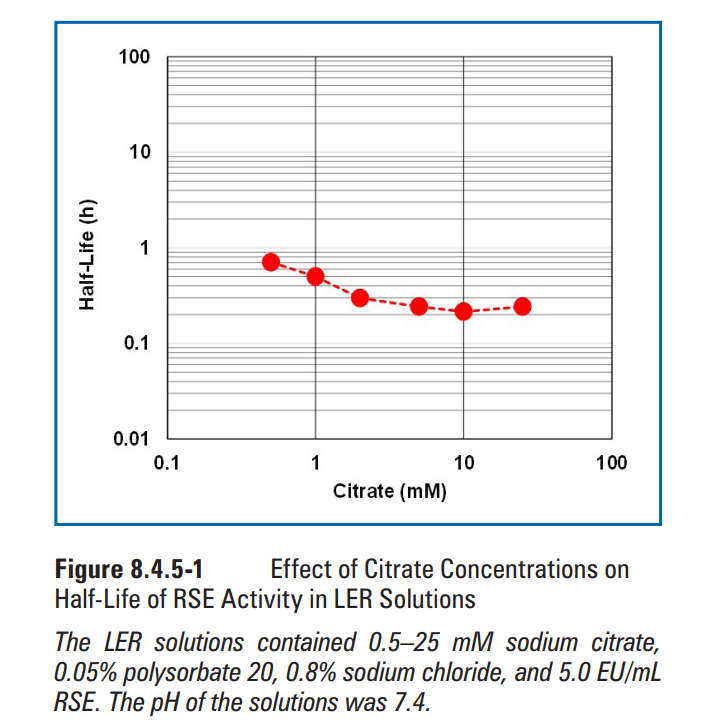
图8.4.5-1柠檬酸盐浓度对LER溶液中RSE活性半衰期的影响 (图片来源于网络,仅供学习交流用)LER溶液含0.5–25 mM柠檬酸钠、0.05%聚山梨酯20、0.8%氯化钠和5.0 EU/mL RSE。溶液的pH值为7.4
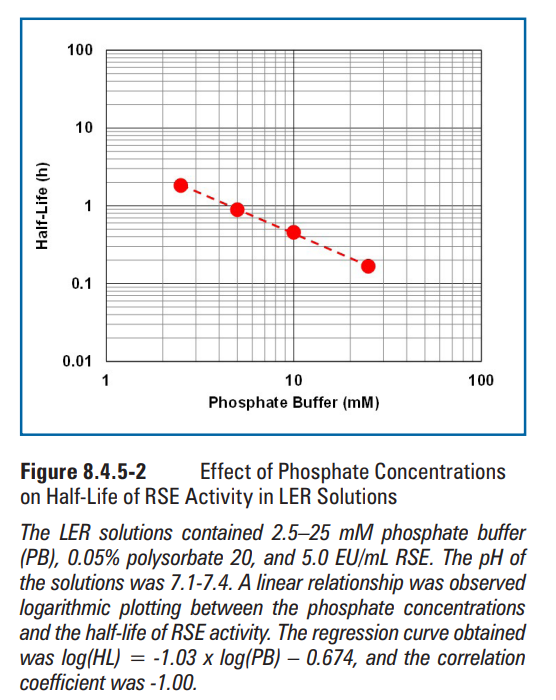
图8.4.5-2磷酸盐浓度对LER溶液中RSE活性半衰期的影响(图片来源于网络,仅供学习交流用) LER溶液含有2.5–25 mM磷酸盐缓冲液(PB)、0.05%聚山梨酯20和5.0 EU/mL RSE。溶液的pH值为7.1 ~ 7.4,磷酸盐浓度与RSE活性半衰期呈线性关系。所得回归曲线为log(HL)=-1.03xlog(PB)-0.674,相关系数为-1.00。
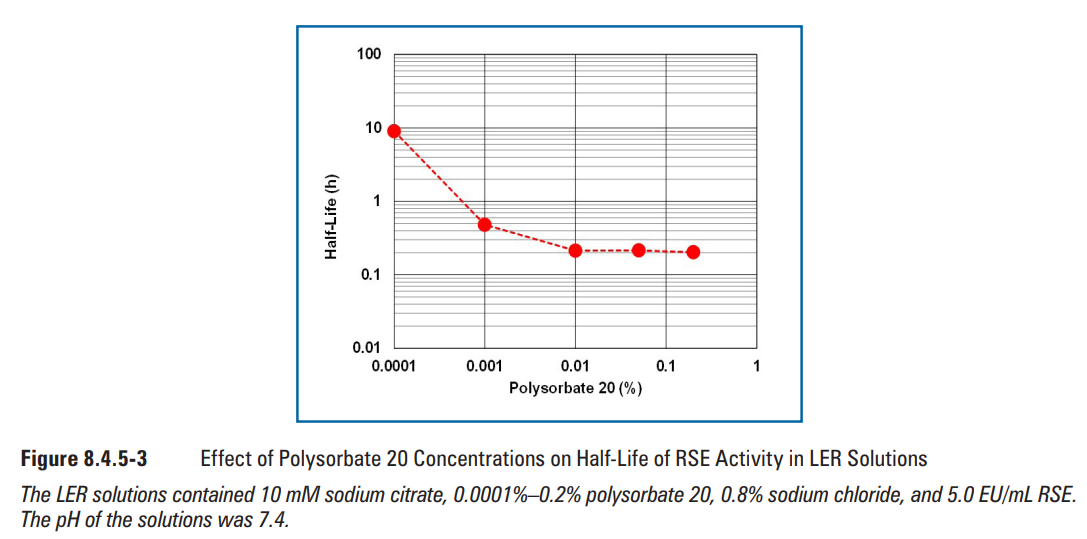
图8.4.5-3聚山梨酯20浓度对LER溶液中RSE活性半衰期的影响(图片来源于网络,仅供学习交流用) LER溶液含10 mM柠檬酸钠、0.0001%-0.2%聚山梨酯20、0.8%氯化钠和5.0 EU/mL RSE。溶液的pH值为7.4。
8.4.6Effects of Dilution Methods on Recovery of RSE in LER Solutions 稀释方法对LER溶液中RSE回收率的影响
RSE activity change in the LER solutions containing 10 mM sodium citrate, 0.05% polysorbate 20, and saline was measured by three different dilution methods—the direct assay method, the water dilution method, and the magnesium dilution method—at 25 oC and 2 oC-5 oC. The RSE activity gradually decreased over time at 25 oC (Figure 8.4.6-1). The RSE activity was lower with the water dilution method than with others after 10 min, even though the compositions of the reaction mixtures were exactly the same as the direct assay and water dilution methods. Similar results were also observed in the LER solution stored at 2 oC-5 oC after one day (Figure 8.4.6-2). These results suggested that RSE aggregates were quickly altered during the dilution with water. Interestingly, the RSE activity was maintained for four days at 2 oC-5 oC with the direct assay method and the magnesium dilution method (Figure 8.4.6-2). RSE activity was also maintained for at least 15 days at 2 oC-5 oC in LER solutions with different compositions (Figure 8.4.6-3). In the direct assay method, RSE aggregates probably reacted with the LAL reagent before the aggregates were altered. The magnesium dilution method probably prevented the alteration of RSE aggregates during the dilution. With the water dilution method, even though the RSE activity in the LER solution decreased in one day, the activity was maintained after 0.02 day (30 min) at low temperature (Figure 8.4.6-2). This indicates that the original RSE aggregates can be maintained for a while at low temperature. Considering these results, there are three phases of RSE in LER solutions:
在25℃和2℃-5℃条件下,通过三种不同的稀释方法(直接测定法、水稀释法和镁离子稀释法)测定含10 mM柠檬酸钠、0.05%聚山梨酯20和生理盐水的LER溶液中RSE活性变化。在25 ℃下,RSE活性随时间逐渐降低(图8.4.6-1)。尽管反应混合物的组成与直接测定法和水稀释法完全相同,但水稀释法的RSE活性在10 min后低于其他方法。在2 ℃-5 ℃条件下储存1天后,在LER溶液中也观察到相似结果(图8.4.6-2)。这些结果表明,在用水稀释期间,RSE聚集体迅速改变。有趣的是,采用直接测定法和镁稀释法时,RSE活性在2℃-5℃条件下可维持4天(图8.4.6-2)。在2℃-5℃条件下,在不同组成的LER溶液中,RSE活性维持了至少15天(图8.4.6-3)。在直接测定方法中,RSE聚集体可能在聚集体改变之前与LAL试剂反应。镁稀释法可能阻止了稀释过程中RSE聚集体的改变。用水稀释法,尽管LER溶液中RSE活性在一天内下降,但在低温下RSE活性仍维持了0.02天(30 min)(图8.4.6-2)。这表明原始的RSE聚集体可以在低温下保持一段时间。考虑到这些结果,LER溶液中存在三个阶段的RSE:
•First phase: RSE activity is high with all three dilution methods.
第一阶段:所有三种稀释方法的RSE活性均较高。
•Second phase: RSE activity is kept in the original LER solutions and is decreased by the dilution with water.
第二阶段:RSE活性保持在原始LER溶液中,并通过用水稀释降低。
•Third Phase: RSE activity is decreased, and all three dilution methods show low activity.
第三阶段:RSE活性降低,三种稀释方法均显示活性较低。
Different compositions of the LER solutions showed different degrees of the reduction of the RSE activ- ity in the LER solutions with the water dilution method (Figure 8.4.6-3). Polysorbate 80 showed less LER effect than polysorbate 20. This suggested that the carbon chain length in the detergent is important in LER effect because lauric acid in polysorbate 20 has a closer carbon chain length to the fatty acids in Escherichia coli Lipid A than oleic acid in polysorbate 80. Citrate showed a stronger LER effect than phosphate. This can be explained by the strength of the chelate effect, as discussed in Section 8.4.5.
不同组成的LER溶液显示,采用水稀释法时,LER溶液中的RSE活性降低程度不同(图8.4.6-3)。聚山梨酯80的LER效应比聚山梨酯20低。这表明洗涤剂中的碳链长度在LER效应中很重要,因为聚山梨酯20中的月桂酸比聚山梨酯80中的油酸更接近大肠埃希菌脂质A中脂肪酸的碳链长度。柠檬酸盐的LER效应强于磷酸盐。这可以通过螯合作用来解释,如第8.4.5节所述。
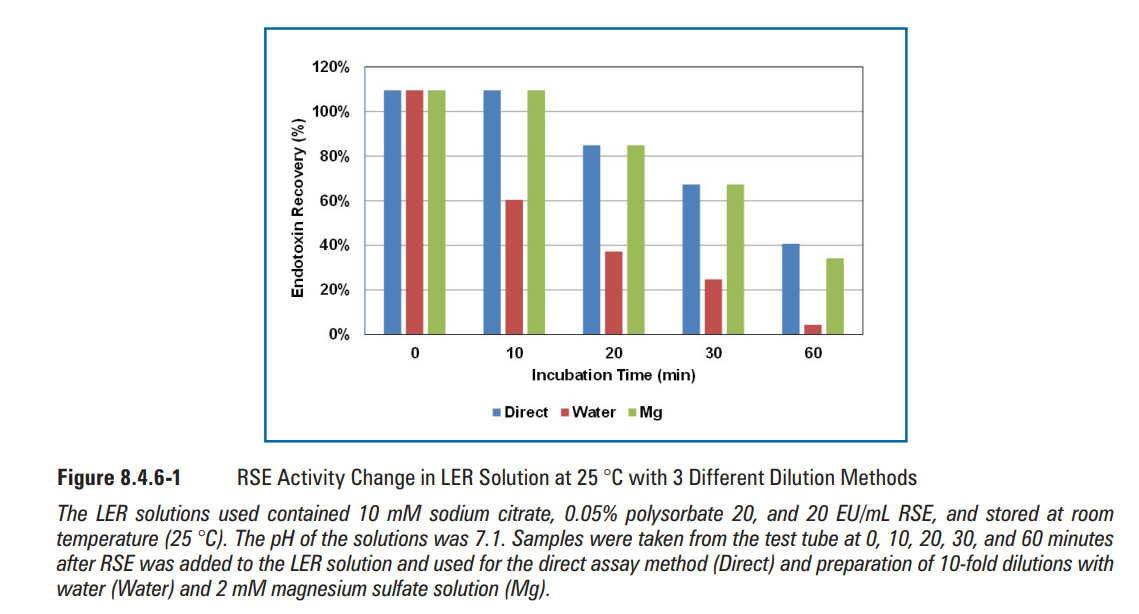
图8.4.6-1采用3种不同稀释方法在25 ℃下LER溶液中的RSE活性变化(图片来源于网络仅供学习交流用)
使用的LER溶液含有10 mM柠檬酸钠、0.05%聚山梨酯20和20 EU/mL RSE,并在室温(25 ℃)下储存。溶液的pH值为7.1。在向LER溶液中加入RSE后0、10、20、30和60 min,从试管中取样,用于直接测定法(直接),并使用水(水)和2 mM硫酸( 硫酸)镁溶液(Mg)制备10倍稀释液。
硫酸)镁溶液(Mg)制备10倍稀释液。
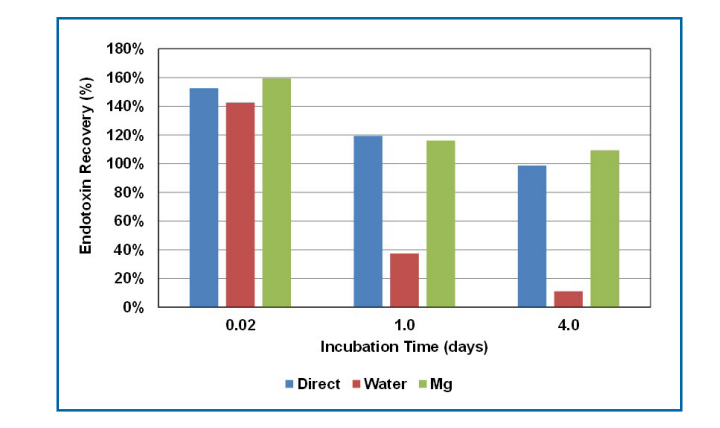
图8.4.6-2采用3种不同稀释方法在2 ℃-5 ℃下LER溶液中的RSE活性变化(图片来源于网络仅供学习交流用)
使用的LER溶液含有10 mM柠檬酸钠、0.05%聚山梨酯20和20 EU/mL RSE,并储存在冰箱中(2 ℃-5 ℃)。溶液的pH值为7.1。向LER溶液中加入RSE后0.02(30 min)、1和4天,从试管中取样,用于直接测定法(直接),并使用水(水)和2 mM硫酸镁溶液(Mg)制备10倍稀释液。
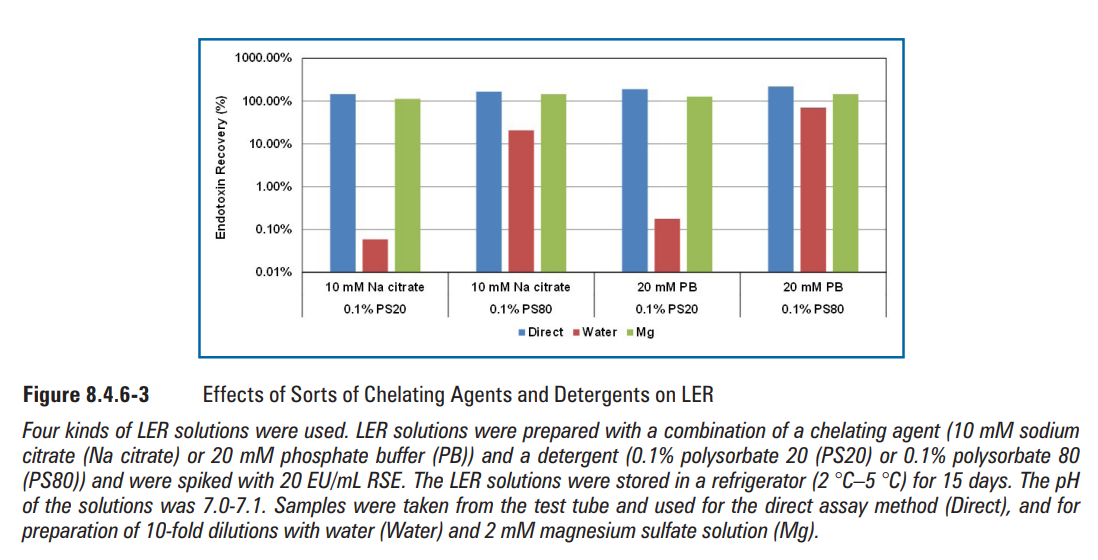
图8.4.6-3各种螯合剂和清洁剂对LER的影响(图片来源于网络仅供学习交流用)
使用了四种LER溶液。使用螯合剂(10 mM柠檬酸钠(柠檬酸钠)或20 mM磷酸盐缓冲液(PB))和洗涤剂(0.1%聚山梨酯20(PS20)或0.1%聚山梨酯80(PS80))组合制备LER溶液,并加标20 EU/mL RSE。将LER溶液在冰箱(2 ℃-5 ℃)中储存15天。溶液的pH值为7.0-7.1。从试管中取样并用于直接测定法(直接),以及用水(水)和2 mM硫酸镁溶液(Mg)制备10倍稀释液。
8.4.7Conditions for Magnesium Dilution Method镁离子稀释方法的条件
The magnesium dilution method showed comparable values to the direct assay method (Figures 8.4.6-1 – 8.4.6-3) indicating that the addition of magnesium prevents the change in RSE aggregation status during the dilution. This method is probably suitable for the measurement of endotoxin activity in original LER solutions. Appropriate magnesium concentrations were examined for this method by using an LER solution containing 10 mM sodium citrate, 0.05% polysorbate 20, and 20 EU/mL RSE in saline stored at 2 oC–5 oC for three days. RSE recovery in 10-fold dilutions was consistently obtained in the range between 100% and 140% with magnesium concentrations between 0.6 mM and 10 mM. The RSE recovery was lower with magnesium concentrations below 0.6 mM, indicating that the magnesium concentration should be equal to or higher than 0.6 mM to neutralize the citrate effect in the diluted LER solutions. The rate for the first dilution with 2 mM magnesium sulfate was compared between 5-fold and 50-fold using the same LER solution, and there was no significant difference in RSE recovery between dilution rates. The average recovery was 119% with the coefficient of variation at 5.1%. The spiked RSE concentration did not affect the RSE recovery in the magnesium dilutions of the LER solutions containing 10 mM sodium citrate and 0.05% polysorbate 20 in the range between 2 EU/mL and 200 EU/mL. These results suggest that the magnesium dilution method using 2 mM magnesium solution is useful to measure endotoxin activity in LER solutions. The results with the magnesium dilution method 1) reproduced the results of Platco, et al., although the magnesium concentrations were different, and 2) support the suggestion that the storage temperature of the samples at 2 oC–8 oC is important (21). Further, the results indicate CSE activity was already decreased in the LER solutions because CSE activity was resurrected by dilution of magnesium sul- fate in Tris buffer. However, the results in the Platco, et al., study indicated that the RSE activity was maintained in the undiluted LER solutions, and that the magnesium dilution method prevented the alteration of RSE aggregates by dilution.
此外,结果表明CSE活性已经 在LER溶液中降低,因为通过在Tris缓冲液中稀释硫酸镁使CSE活性复活。然而,Platco等人的研究结果表明,在未稀释的LER溶液中保持了RSE活性,并且镁稀释法通过稀释防止了RSE聚集体的改变。
镁稀释法的测定值与直接测定法相当(图8.4.6-1-8.4.6-3),表明镁的加入阻止了稀释过程中RSE聚集状态的变化。该方法可能适用于测定LER原液中内毒素活性。用含有10 mM柠檬酸钠、0.05%聚山梨酯20和20EU/mL RSE的LER溶液在2℃-5℃的生理盐水中储存3天,以确定该方法的适宜镁浓度。当镁浓度为0.6 mM至10 mM时,10倍稀释的RSE回收率始终在100%至140%之间。当镁浓度小于0.6 mM时,RSE回收率较低,表明镁浓度应等于或大于0.6 mM,可中和稀释LER溶液中的柠檬酸盐效应。用相同的LER溶液将2 mM硫酸镁一步稀释(一次稀释到位)至5倍和50倍进行比较,两种稀释倍数之间的RSE回收率没有显著差异。平均回收率为119%,变异系数为5.1%。在含10 mM柠檬酸钠和0.05%聚山梨酯20的LER溶液中,添加的RSE浓度在2EU/mL到200EU/mL的范围内不影响RSE在镁的稀释度中的回收率。这些结果表明,用2 mm镁溶液的镁稀释法测定LER溶液中的内毒素活性是可行的。镁稀释法的结果1) 尽管镁浓度不同,该实验重现了Platco等人的结果;2)支持样品在2 ℃-8℃下储存温度的建议 (21)。此外,结果表明CSE活性已经在LER溶液中降低,因为通过在Tris缓冲液中稀释硫酸镁使CSE活性重新恢复。然而,Platco等人的研究结果表明,在未稀释的LER溶液中保持了RSE活性,并且镁稀释法在稀释过程中防止了RSE聚集体的改变
8.4.8Conclusion结论
LER was considered a temperature-dependent chemical reaction affected by pH, salt concentrations, and components of LER matrices. Since the half-life of RSE activity in LER solutions showed a linear relationship with temperature, pH, and salt concentration, information on the half-life is useful to anticipate the LER effects in pharmaceutical products. Lower temperature, lower pH, and higher salt concentrations are recommended to avoid LER.
LER被认为是受pH值、盐浓度和LER基质组分影响的温度依赖性化学反应。由于LER溶液中RSE活性的半衰期与温度、pH值和盐浓度呈线性关系,因此半衰期信息有助于预测药品中的LER效应。建议降低温度、降低pH值和升高盐浓度,以避免LER。
Since the BET and the rabbit pyrogen test are bioactivity assays, they do not determine the physical concentrations of LPS. Therefore, the physical weight of LPS in the samples cannot be determined using the BET, based on the LAL test. The potency of RSE or CSE expressed in endotoxin units per nanogram (EU/ng) is known, but the potency of contaminated endotoxin in samples is unknown; only the endotoxin activity in the sample is known. ftere is an LPS aggregation status the potency of which is very low under LER conditions. Because the biological activity and clinical risks of undetectable LPS under LER conditions are unknown, further studies are necessary. This subject is different from the target of the BET, which measures only endotoxin activity in products. Therefore, the LER issue is not the BET issue; it is a new matter to be investigated. Considering that LER is the change in potency of RSE or CSE and does not reflect the stability of contaminated endotoxin in products, the current design for hold-time studies using purified LPS may not be suitable. For the future inves- tigation of LER, an understanding of LER mechanism will be important, and the information on the kinetically analyzed factors affecting LER will be helpful.
由于BET和家兔热原试验是生物活性试验,因此它们不能确定细菌内毒素的物理浓度。因此,基于LAL试验,无法使用BET测定样本中脂多糖(LPS)的物理重量。以内毒素单位/纳克(EU/ng)表示的RSE或CSE的效价是已知的,但样品中污染内毒素的效价未知;仅已知样品中的内毒素活性。有一种LPS聚集状态,其效价在LER条件下效力很低。由于在LER条件下无法检测到LPS的生物活性和临床风险尚不清楚,因此有必要进行进一步研究。该主题与BET的目标不同,BET仅测量产品中的内毒素活性。因此,LER问题不是BET问题;这是一个需要调查的新问题。考虑到LER是RSE或CSE效价的变化,并不能反映产品中污染内毒素的稳定性,因此使用纯化LPS进行放置时间研究的当前设计可能不适用。对于未来LER的研究,了解LER机制将是很重要的,关于影响LER的动力学分析因素的信息将是有帮助的。
There seem to be three phases in LER solutions. The first phase is a stage progressing chelation on the LPS aggregates, though the LPS aggregates are still strong enough to keep the activity in the water dilutions. In the second phase, the chelation on the surface LPS probably proceeds quickly, but the LPS aggregates are still maintained in the solution. The activity is decreased by the water dilution, but the magnesium dilution can maintain the LPS aggregates to show activity. The second phase can be maintained for a long time at a low temperature. In the third phase, significant amounts of the surface LPS molecules may be replaced with detergent molecules, and the biological activity is lost. Even the direct assay and magnesium dilution methods cannot detect the original levels of RSE activity in the LER solutions in this phase. Considering this mechanism, controlling the temperature of samples and using the magnesium dilution method for hold-time studies is important.
LER解决方案似乎分为三个阶段。第一阶段是在脂多糖(LPS)聚集体上进行逐步螯合的阶段,脂多糖(LPS)聚集体仍然足够强以保持水稀释中的活性。在第二阶段,表面脂多糖(LPS)的螯合可能进一步加快,但内毒素聚集体仍保持在溶液中。水稀释会降低酶的活性,而镁的稀释能维持脂多糖聚集体的活性。第二相可在较低温度下保持较长时间。在第三阶段,大量的表面脂多糖(LPS)分子可能被洗涤剂分子取代,生物活性丧失。即使是直接测定法和镁稀释法也不能检测到这一阶段LER溶液中RSE活性的原始水平。考虑到这一机理,控制样品的温度并使用镁稀释法进行保温研究是很重要的。
Acknowledgements致谢
The author thanks Dr. Jack Levin and Dr. James F. Cooper for their thoughtful suggestions.
感谢Jack Levin博士和James F. Cooper博士深思熟虑的建议。
8.4.9References参考文献
1.Limulus Test and its Application (in Japanese). Tsuchiya, M. 1990, J Antibact Antifung Agents, Vol. 18, pp. 287-294.
2.Effects of Iron on Bacterial Endotoxin. Roth, R; et al., 1997, J Endotoxin Res., Vol. 4(4), pp. 273-278.
3.Saline and Buffers Minimize the Action of Interfering Factors in the Bacterial Endotoxins Test. Fujita, Y; et al., 2011, Anal Biochem., Vol. 409(1), pp. 46-53.
4.The use of endotoxin as an analyte in biopharmaceutical product hold time study. Bolden, J; et al., 2015, Phar- macopeial Forum, Vol. 41(5).
5.Low Endotoxin Recovery in Common Biologics Products. Chen, J and Vinther, A. Presented at the 2013 PDA Annual Meeting, Orlando, FL.
6.Possible Mechanism of Low Endotoxin Recovery. Tsuchiya, M.2014, Am Pharm Rev, Vol. 17(7), pp. 18-23.
7.Masking of endotoxin in surfactant samples: Effects on Limulus-based detection systems. Reich, J, et al., 2016, Biologicals, Vol. 44, pp. 417-422.
8.Endotoxin Recovery Using Limulus Amebocyte Lysate (LAL) Assay. Bolden, J; et al., 2016, Biologicals, Vol. 44(5), pp. 434-440.
9.Resolution of Low Endotoxin/Lipopolysaccharide Recovery (LER/LLR) Testing. Platco, C; et al., 2016, Am Pharm Rev, Vol. 19(5), pp. 20-25.
10.Data Based Mechanism of Low Endotoxin Recovery (LER). Tsuchiya, M. Presented at the PDA 12th Annual Global Conference on Pharmaceutical Microbiology, Bethesda, MD, October 2017.

