小编推荐:提供一下服务
项目立项调研;进口标准和申报资料;MAH落地的制剂原料厂对接;批件及技术转让对接
联系电话:聂先生15249406857(微信同号)
1.氯卡色林简介
氯卡色林是一种5-HT2C 受体激动剂,诱发饱腹感,降低食欲,减少进食从而达到帮助患者减重的目的。该药由Arena开发,于2012年06月Belviq速释片获得FDA批准,2016年07月Belviq XR缓释片获得FDA批准上市。2017年卫材和Arena达成合作协议,以4900万美元的价格转让氯卡色林的权益。2020年2月卫材宣布将Belviq和撤出市场,并停止在美国市场销售。
2018年7月31日,CY Biotech获得卫材Belviq在中国(包括台湾和香港)的独家开发和销售权。
2.国内仿制情况
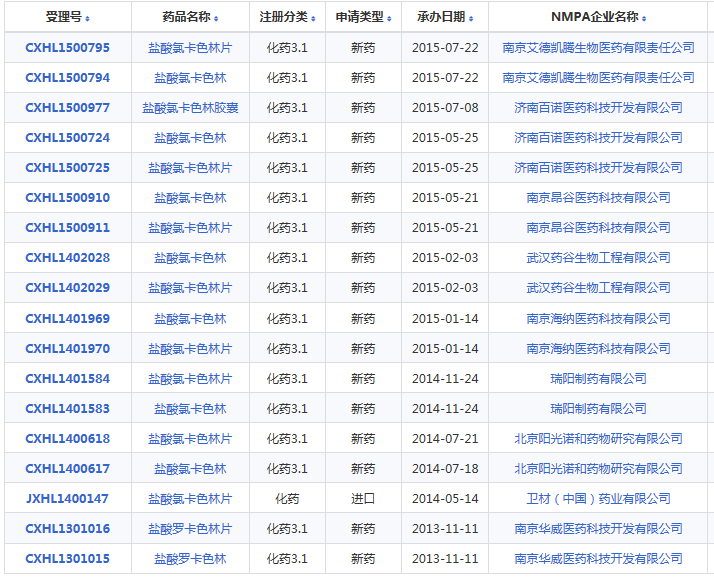
卫材已经同意将Belviq和Belviq XR从美国上市退出,氯卡色林目前只在美国上市,但在CDE官网查询,有三个相关临床试验,主要涉及2个药企:康缘华威和瑞阳制药。氯卡色林从美国退市必将影响仿制药的审批结果,国内两家仿制药企业都已经完成了生物等效性(BE)试验,若最终受到影响,投入的研发费用将打水漂。
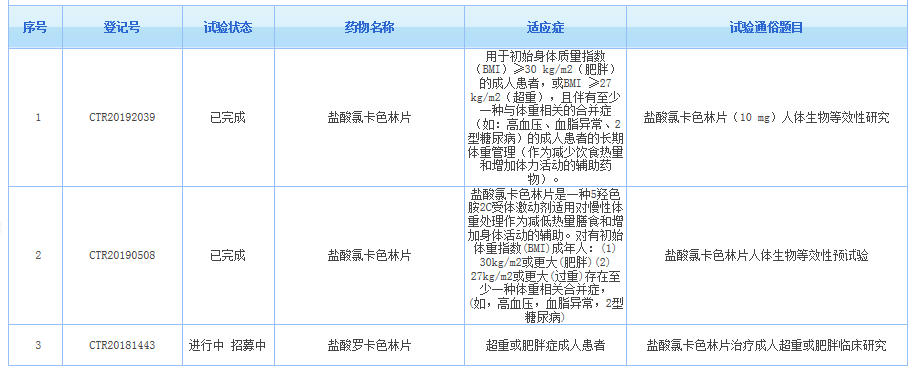
小编又在药智网上查询了一下,在2016年之前有以下企业申报了氯卡色林,这些企业该品种的仿制项目也将受到影响,考虑到在7.22之前做的,投入有限,影响较小:
3.FDA要求氯卡色林退市信息

What safety cocern is FDAannouncing?
The U.S. Food and Drug Administration (FDA) has requestedthat the manufacturer of Belviq, Belviq XR (lorcaserin) voluntarily withdrawthe weight-loss drug from the U.S. market because a safety clinical trial showsan increased occurrence of cancer. The drug manufacturer, Eisai Inc,. hassubmitted a request to voluntarily withdraw the drug.
美国食品药品监督管理局(FDA)因安全的临床试验显示癌症的发生率增加,要求Belviq, Belviq XR(lorcaserin)的制造商自愿从美国市场撤回该减肥药物。 药品制造商卫材公司已提交了自愿撤药的请求。
What is FDA doing?
We are taking this action because we believe that therisks of lorcaserin outweigh its benefits based on our completed review ofresults from a randomized clinical trial assessing safety.
In January 2020, we announced we were reviewing clinical trial data andalerted the public about a possible risk of cancer associated with lorcaserinbased on preliminary analysis of the data.
我们之所以采取此行动是因为我们认为,根据我们对安全性评估的随机临床试验结果的完整审查,lorcaserin的风险大于其获益。
在2020年1月,我们曾宣布我们正在审查临床试验数据,并基于对数据的初步分析,向公众警告了与lorcaserin相关的癌症风险。
Whatshould patients do?
Patients should stop taking lorcaserin and talk to yourhealth care professionals about alternative weight-loss medicines and weightmanagement programs. It’s best to dispose ofunused lorcaserin using a drugtake back location, but if you can’tget to one you can dispose of lorcaserin in your household trash:
1. Mix the pills with an unappealing substance such as dirt,cat litter, or used coffee grounds; do not crush them.
2. Place the mixture in a container such as a sealed plasticbag.
3. Throw away the container in your trash at home.
4. Remove or delete all personal information on theprescription label of empty medicine bottles or packaging, then throw away orrecycle them.
FDA is not recommending special screening for patientswho have taken lorcaserin. Talk to your health care professional if you havequestions.
患者应停止服用氯卡色林,并与您的医疗保健专业人员讨论替代减肥药和体重管理计划。最好使用药品回收处来处理未使用的氯卡色林,但是如果您无法做到这一点,则可以将氯卡色林丢入家庭垃圾中:
1.将药丸与诸如污垢,猫砂或用过的咖啡渣等不吸引人的物质混合;不要粉碎他们。2.将混合物放入密封的塑料袋等容器中。3.将容器丢到家里的垃圾桶中。4.删除或删除空药瓶或包装的处方标签上的所有个人信息,然后丢弃或回收它们。
FDA不建议对服用氯卡色林的患者进行特殊筛查。如有疑问,请咨询您的医疗保健专业人员。
Whatshould health care professionals do?
Health care professionals should stop prescribing anddispensing lorcaserin to patients. Contact patients currently takinglorcaserin, inform them of the increased occurrence of cancer seen in theclinical trial, and ask them to stop taking the medicine. Discuss alternativeweight-loss medicines or strategies with your patients.
FDA is not recommending special screening for patientswho have taken lorcaserin. As with any individual patient, regardless of priorlorcaserin treatment, standard screening recommendations for cancer should be implemented.
卫生保健专业人员应停止向患者开处方和分配氯卡色林。 与目前正在服用氯卡色林的患者联系,告知他们在临床试验中发现的癌症发生率增加,并要求他们停止服药。与您的患者讨论替代减肥药物或策略。
FDA不建议对服用氯卡色林的患者进行特殊筛查。与任何个体患者一样,无论先前是否使用氯卡色林治疗,都应实施标准的癌症筛查建议。
Whatdid FDA find?
When FDA approved lorcaserin in 2012, we required thedrug manufacturer to conduct a randomized, double-blind, placebo-controlledclinical trial to evaluate the risk of cardiovascular problems, which foundthat more patients taking lorcaserin (n=462; 7.7 percent) were diagnosed with cancercompared to those taking a placebo, which is an inactive treatment (n=423; 7.1percent). The trial was conducted in 12,000 patients over 5 years. A range ofcancer types was reported, with several different types of cancers occurringmore frequently in the lorcaserin group, including pancreatic, colorectal, andlung.
当FDA在2012年批准氯卡色林时,我们要求药物制造商进行一项随机,双盲,安慰剂对照的临床试验,以评估心血管疾病的风险。该试验在5年内对12,000名患者进行了研究,结果发现服用氯卡色林的患者与服用安慰剂者相比更多:氯卡色林组(n = 462; 7.7%),安慰剂组(n= 423; 7.1%)。 据报道,癌症的类型范围很广,在氯卡色林组中,几种不同类型的癌症更常见,包括胰腺癌,结直肠癌和肺癌。
Howdo report side effects from lorcaserin?
To help FDA track safety issues with medicines, we urgepatients and health care professionals to report side effects involvinglorcaserin or other medicines to the FDA MedWatch program, using the informationin the “Contact FDA” box at the bottom of the page.
为帮助FDA追踪药物的安全性问题,我们敦促患者和卫生保健专业人员使用页面底部“联系FDA”框中的信息,向FDA药品监督部门报告涉及氯色林或其他药物的副作用。
Datasummary
We reviewed data from the Cardiovascular and MetabolicEffects of Lorcaserin in Overweight and Obese Patients – Thrombolysis inMyocardial Infarction 61 (CAMELLIA-TIMI 61) clinical trial. It was arandomized, double-blind, placebo-controlled, multicenter, parallel group trialconducted between January 2014 and June 2018 in the U.S., Canada, Mexico, theBahamas, Europe, South America, Australia, and New Zealand. The studypopulation consisted of 12,000 men and women who were overweight or obese.Patients were required to have either established cardiovascular disease, or tobe at least 50 years old for men or 55 years for women with type 2 diabetesmellitus plus at least one additional cardiovascular risk factor. Eligiblepatients were assigned randomly to either lorcaserin 10 mg twice daily orplacebo. Approximately 96 percent of patients completed the study, and 62percent who completed remained on treatment at the end of study. The medianfollow-up time was 3 years and 3 months.
The primary safety analysis showed no meaningfuldifference between lorcaserin and placebo in the risk of major adversecardiovascular events, demonstrating noninferiority. The one-sided upper boundof the 95% confidence interval (CI) of the hazard ratio (HR) was less than 1.4(the noninferiority margin). The HR (95% CI) was 1.005 (0.842, 1.198) forlorcaserin versus placebo.
There was a numerical imbalance in the number of patientswith malignancies, with one additional cancer observed per 470 patients treatedfor one year. During the course of the trial, 462 (7.7 percent) patientstreated with lorcaserin were diagnosed with 520 primary cancers compared to theplacebo group, in which 423 (7.1 percent) patients were diagnosed with 470cancers. Imbalances in specific cancers including pancreatic, colorectal, andlung contributed to the observed overall imbalance in cancer cases. There wasno apparent difference in the incidence of cancer over the initial months oftreatment, but the imbalance increased with longer duration on lorcaserin.
我们回顾了氯卡色林对超重和肥胖患者的心血管和代谢作用的数据-心肌梗塞61(CAMELLIA-TIMI61)的溶栓治疗临床试验。 这是一项于2014年1月至2018年6月在美国,加拿大,墨西哥,巴哈马,欧洲,南美,澳大利亚和新西兰进行的随机,双盲,安慰剂对照,多中心,平行组试验。研究人群包括超重或肥胖的12,000名男性和女性。 患者必须患有心血管疾病,或者男性至少50岁或女性至少55岁的患有2型糖尿病加上至少一种心血管危险因素。符合条件的患者被随机分配,每天两次使用氯卡色林10 mg或安慰剂。 大约96%的患者完成了研究,而62%的完成研究的患者在研究结束时仍在接受治疗。 中位随访时间为3年3个月。
初步安全性分析显示,氯卡色林组和安慰剂组在重大不良心血管事件发生风险方面没有有意义的差异,这表明对照组具有非劣效性。安慰剂组的95%置信区间的一侧上限小于1.4(非劣质性余量)。安慰剂组相较于氯卡色林组(95%置信区间)为1.005(0.842,1.198)。
在恶性肿瘤患者数量上存在数字上的不平衡,氯卡色林治疗一年,每470名患者观察到一种癌症。在试验过程中,与安慰剂组相比,接受氯卡色林治疗的462名患者(7.7%)被诊断出患有520例原发性癌症,而安慰剂组中有423名患者(7.1%)被诊断出患有470例癌症。特定癌症(包括胰腺癌,结肠直肠癌和肺癌)的失衡导致了在癌症病例中观察到的总体失衡。在治疗的最初几个月中,癌症的发生率没有明显差异,但是随着氯卡色林持续时间的延长,这种失衡会加剧。

